India’s pharmaceutical manufacturing sector is a cornerstone of global healthcare. As the world’s largest supplier of generic medicines and a critical hub for vaccine production, Indian pharma organizations hold immense economic, strategic, and public health significance.
But this global importance has also made the sector a prime cyber target.
In 2024-2025, Indian pharmaceutical manufacturers are navigating a markedly different cybersecurity landscape. Industry trends reflect a convergence of high-value intellectual property, deeply embedded legacy operational technology (OT), accelerated digital transformation initiatives, and a more stringent regulatory environment shaped by the DPDP Act 2023 and global data integrity expectations.
This blog examines the evolving threat landscape facing Indian pharma manufacturing and explains why a unified security approach is no longer optional.
Indian pharma manufacturers operate in an environment where cyber incidents are no longer confined to IT systems. Digital transformation initiatives, often referred to as Pharma 4.0, are connecting decades-old shop-floor systems to enterprise IT networks and cloud platforms.
While this improves efficiency and visibility, it also creates a vastly expanded attack surface, one where a single breach can impact:
The result is a sector where cybersecurity failures translate directly into financial, operational, and reputational damage.
For pharma companies, IP is everything, drug formulations, process chemistry, clinical trial data, and proprietary manufacturing techniques.
The threat:
Sophisticated attackers, including corporate competitors and nation-state actors, are conducting long-dwell, low-and-slow intrusions designed to quietly exfiltrate R&D data. These attacks often go undetected for months, undermining competitive advantage and global market position.
Ransomware attacks against pharma manufacturers are no longer limited to email servers or file shares.
The new attack path:
Threat actors breach IT systems and then move laterally into OT environments, targeting HMIs, historians, and production control systems. Encrypting these assets can halt production lines entirely.
The impact:
Even a single day of downtime can cost millions, disrupt drug availability, and create immense pressure to pay ransoms, especially during critical manufacturing cycles.
Cyber risk has become a direct regulatory risk.
In today’s environment, a cyber incident can instantly become a compliance crisis.
Pharma manufacturing plants rely heavily on equipment designed to run for 20–30 years, often on obsolete operating systems that cannot be patched or instrumented with modern security agents.
At the same time, organizational silos persist:
This divide creates blind spots that attackers exploit, especially at the IT/OT boundary.
Traditional security approaches, separate tools for IT security, OT monitoring, and compliance reporting, are failing pharma manufacturers.
They:
What Indian pharma organizations need instead is unified visibility, intelligence, and response across the entire IT/OT environment.
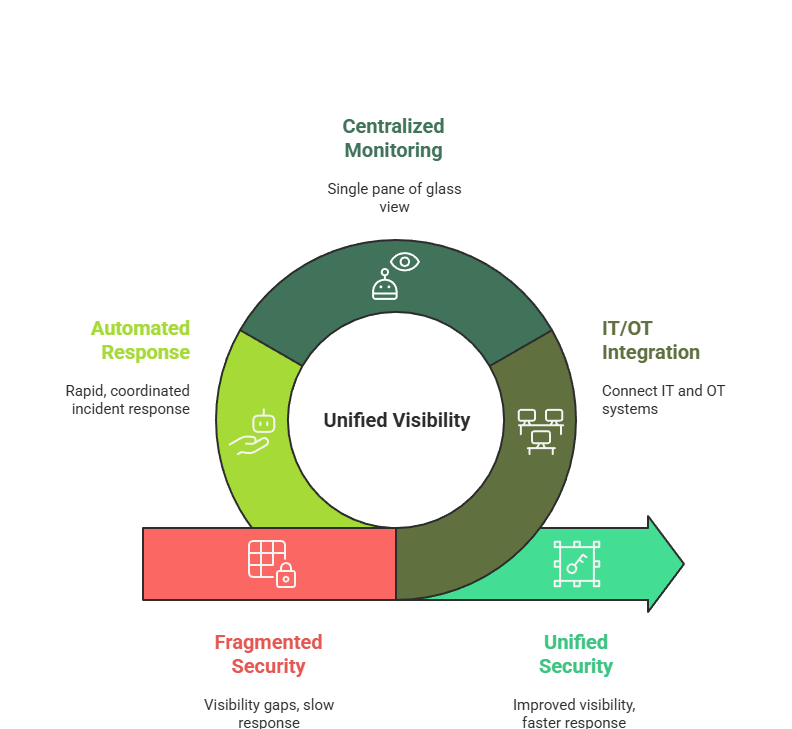
Seceon’s Unified Platform (aiSIEM, aiXDR, and SOAR) directly addresses the realities of Indian pharma manufacturing by delivering security that is agentless, behavior-driven, automated, and compliance-aware.
Signature-based tools cannot detect insider threats or subtle data manipulation.
The Seceon advantage:
User and Entity Behavior Analytics (UEBA) establishes baselines for scientists, engineers, and systems. Anomalous access patterns, unusual file movements, or unauthorized data modifications are detected in real time, protecting IP and ensuring production data integrity.
Validated OT systems and legacy PLCs cannot run endpoint agents without risking compliance or failure.
The Seceon advantage:
Agentless Network Traffic Analysis (NTA) passively monitors OT environments, profiles devices, and detects abnormal communications, such as a legacy machine attempting outbound connections, without disrupting operations.
In pharma manufacturing, minutes matter.
The Seceon advantage:
Integrated SOAR enables sub-second automated response. Compromised engineering workstations or malicious lateral movement attempts can be isolated instantly, preventing ransomware from reaching production systems and avoiding catastrophic downtime.
Audits from CDSCO, US FDA, or international partners demand proof, not promises.
The Seceon advantage:
Centralized logging, continuous monitoring, and automated audit trails across IT and OT environments make it easier to demonstrate due diligence, data protection, and compliance with DPDP Act requirements and global data integrity standards.
| Indian Pharma Challenge | Seceon Unified Platform Relevance |
| Theft of IP and R&D Data | UEBA detects anomalous access, insider threats, and covert exfiltration |
| Ransomware Halting Production | SOAR enables automated, sub-second containment |
| Legacy OT with No Agents | Agentless NTA secures unpatchable systems |
| DPDP Act Compliance | Centralized logging and monitoring for audit readiness |
| Regulatory Data Integrity | Unified visibility and tamper-proof audit trails |
Indian pharma manufacturers are at a crossroads.
As digital transformation accelerates and regulatory scrutiny intensifies, cybersecurity must evolve from a supporting function into a core pillar of operational resilience and global trust.
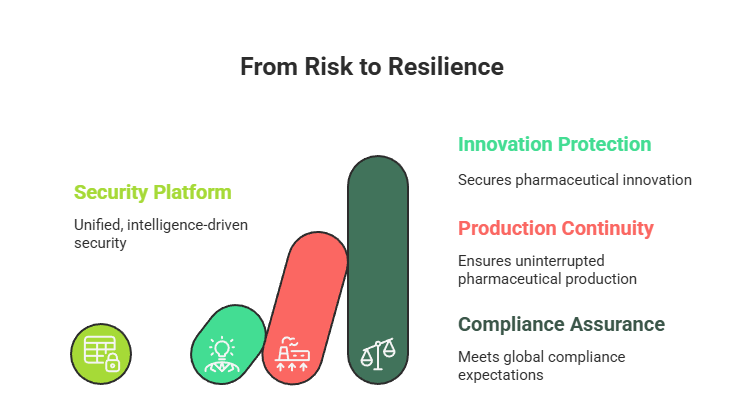
A unified, intelligence-driven security platform is no longer a nice to have, it is essential for protecting innovation, ensuring uninterrupted production, and meeting global compliance expectations.
With unified visibility, automated response, and compliance-ready intelligence, Indian pharma organizations can continue to innovate securely, while protecting their role as the Pharmacy of the World.